The European Union is known for having one of the world’s most rigorous and respected food safety frameworks. The proposed approval of NMN sends a powerful signal to global markets and may serve as a regulatory reference for other jurisdictions.
As a global supplier of high-quality NMN ingredients, XI’AN SPRINGJIA BIO-TECHNIQUE CO., LTD welcomes this development and is fully committed to aligning with international food regulations to better serve our global partners.
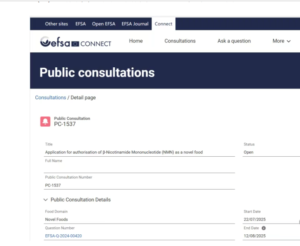
Key implications include:
✅ Full access to the EU market: A clear and compliant path for exports to Europe
✅ Boosted global confidence: A strong endorsement that could influence regulatory decisions in other countries
✅ Industry development catalyst: Encourages further investment in innovation, quality, and scientific validation
NMN: From Research Spotlight to Regulatory Recognition
NMN has gained increasing attention worldwide for its potential in cellular energy metabolism and healthy aging. As the regulatory landscape becomes more defined, NMN is steadily transitioning from the realm of research into real-world nutritional applications across global markets.
XI’AN SPRINGJIA BIO-TECHNIQUE CO., LTD has long focused on the development, production, and quality control of NMN and other advanced nutraceuticals, and is ready to support global brands in leveraging this opportunity.
Looking Ahead
NMN is entering a “golden window” of regulatory and commercial opportunity. The EU’s positive stance not only reinforces confidence in its use, but also paves the way for broader international adoption.
XI’AN SPRINGJIA BIO-TECHNIQUE CO., LTD will continue to uphold our commitment to science-driven innovation, global compliance, and premium-quality manufacturing — supporting the future of health and longevity worldwide.
